Treatment & Management
Approved and Investigational Treatments for GEA
Timely treatment is important in oncology, but a recent study has shown that in patients with advanced HER2-overexpressing GEA, overall and progression-free survival rates are higher in patients treated with an anti-HER2 therapy versus those that did not receive targeted therapy, regardless of treatment initiation timing.1
A dynamic tumor genomic profiling can be used to guide treatment decisions in GEA by understanding the molecular peculiarities that are driving the metastases, as baseline spatial and temporal heterogeneities of targets (eg, HER2 expression) can be a cause of failure/resistance of targeted/immunotherapies. For instance, an Italian study has shown that acquired resistance to trastuzumab-based first-line treatment is the loss of the HER2 receptor.2 As seen in the Phase II PANGEA trial, which stratified and treated patients with GEA based on their tumor characteristics (MSI-high, PD-L1 CPS>10, HER2-positive, etc), it is important to understand the impact of spatial (primary site vs metastasis) and temporal (over time) heterogeneity before commencing treatment, and a new tumor biopsy at the time of progression is extremely relevant.3
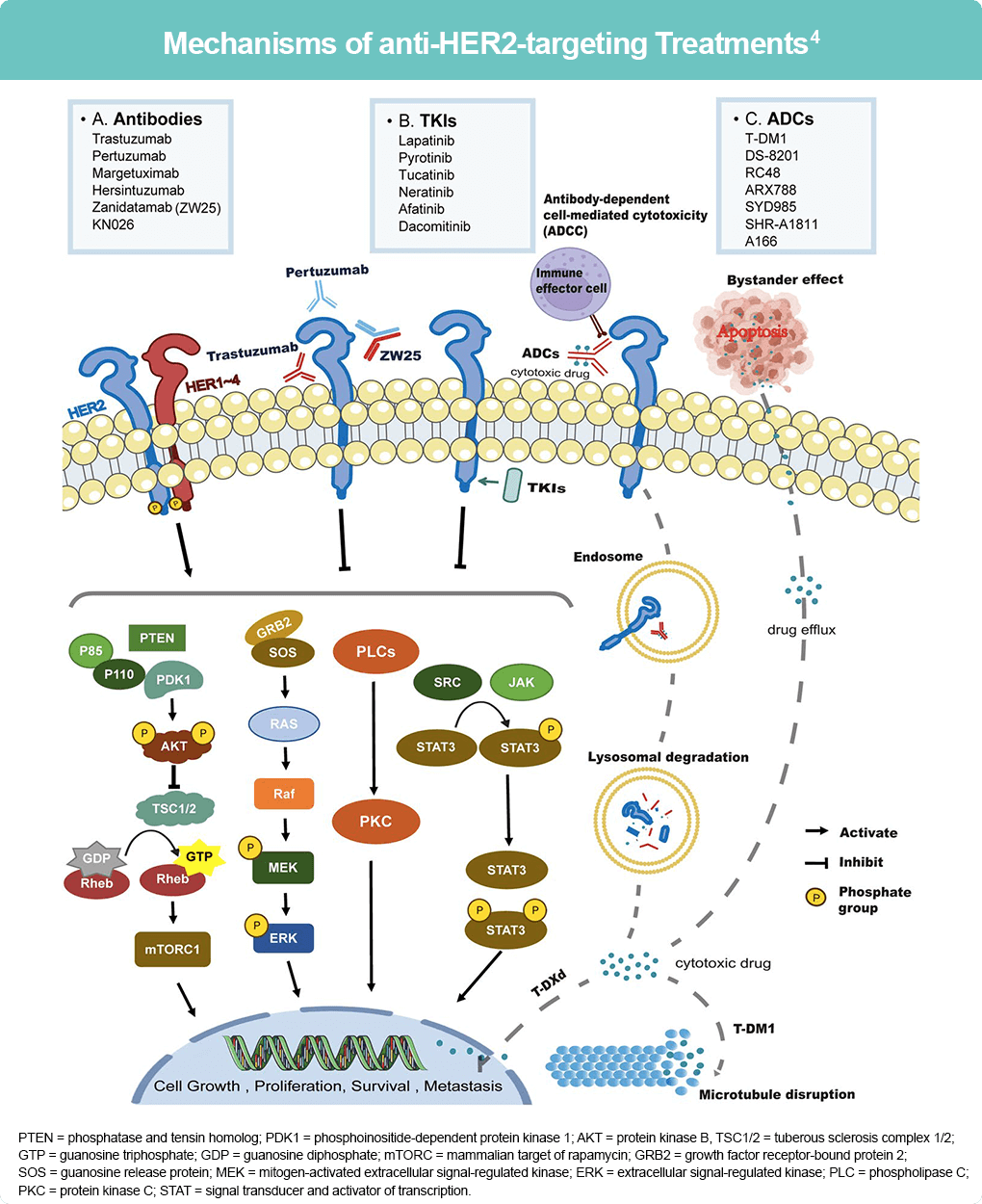
The current classification of anti-HER2 drugs includes antibodies, tyrosine kinase inhibitors (TKIs) and antibody-drug conjugates (ADCs). Antibodies act by binding to the extracellular domain of the HER2 protein and preventing the formation of HER2-containing heterodimers thereby modulating the downstream effectors of HER2 signaling, and by recruiting extracellular immune cells for antibody-dependent cell-mediated cytotoxic effects. Examples include trastuzumab, pertuzumab, and zanidatamab (ZW25).4
PTEN = phosphatase and tensin homolog; PDK1 = phosphoinositide-dependent protein kinase 1; AKT = protein kinase B,
TSC1/2 = tuberous sclerosis complex 1/2; GTP = guanosine triphosphate; GDP = guanosine diphosphate; mTORC = mammalian target of rapamycin; GRB2 = growth factor receptor-bound protein 2; SOS = guanosine release protein; MEK = mitogen-activated extracellular signal-regulated kinase; ERK = extracellular signal-regulated kinase; PLC = phospholipase C; PKC = protein kinase C; STAT = signal transducer and activator of transcription.
TKIs block phosphorylation of tyrosine kinase in the PI3K/AKT and MAPK pathways that are responsible for regulating cell proliferation, migration, angiogenesis, and apoptosis. ADCs are composed of three components: an antibody, a linker, and a cytotoxic molecule. Once the antibody binds to the HER2 protein, the molecules undergo endocytosis and cleavage within the cell, releasing the cytotoxic component that causes damage, among other structures, to the DNA thereby inhibiting cell growth, proliferation, survival, and development of cancer cells.4
Dual-agent HER2 inhibition (eg, HER2-targeting agent plus chemotherapy is also showing promising pathological complete response rates and disease-free survival based on an analysis of 17 studies conducted by Stroes CI, et al in 2021.5
Antibodies
Trastuzumab
Trastuzumab is an anti-HER2 humanized monoclonal antibody approved for first line use in HER2-overexpressing GEA in 2010 based on the results of the phase III ToGA study which met its primary endpoint of significantly improving median overall survival with trastuzumab plus chemotherapy versus chemotherapy alone in the intent-to-treat population (13.8 months vs 11.1 months).6 The HELOISE trial failed to demonstrate a clinical benefit of using a higher dose trastuzumab 10 mg/kg every 3 weeks) plus chemotherapy in HER2-positive patients versus the standard dose of 8 mg/kg loading dose followed by 6 mg/kg every 3 weeks. As a result, the lower dose remains the standard of care for the first line of treatment of HER2-positive GEA.7
Results from the KEYNOTE-811 phase III clinical trial have shown evidence of an improved ORR of addition of PD-1 inhibitor pembrolizumab to a combination treatment of trastuzumab and chemotherapy (fluoropyrimidine and platinum-based molecule) (74.4% vs 51.9%) compared with placebo in HER2 positive GEA. The addition of pembrolizumab to chemotherapy was also deemed to be safe with toxicities comparable in both arms. This benefit is specifically seen in patients with tumors with a PD-L1 combined positive score of 1 or more. Results from overall survival are expected at the end of the trial period, but interim results suggest a possible role for a combination of pembrolizumab and chemotherapy plus targeted therapy in HER2-positive GEA.8, 9 As a result, pembrolizumab in combination with trastuzumab and chemotherapy has gained approval for the treatment of patients with HER2-positive locally advanced unresectable or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma.
A phase Ib/II clinical trial showed that a combination of trastuzumab with ramucirumab, a VEGF-2 targeting agent, improved the prognosis of patients with gastric cancer (overall response rate of 33% and median progression free survival of 7.2 months) who had previously been on trastuzumab treatment alone.10
Pertuzumab
Pertuzumab is a monoclonal antibody that targets the heterodimerization domain of HER2 thereby preventing the heterodimerization of HER2/HER2 receptors and downstream signaling.11 A phase III trial, INNOVATION, is currently studying the efficacy of using trastuzumab/trastuzumab+pertuzumab combination during the perioperative phase after phase II results demonstrated an increased pathologic response rate from 25% to 45%.12
Margetuximab
Margetuximab is a monoclonal Fc-engineered anti-HER2 antibody with a high affinity for CD16A (whose expression is seen in many kinds of immune cells) that is being explored mainly in combination with immunotherapy.9 The phase II/III MAHOGANY trial investigated margetuximab when combined with retifanlimab, an anti-programmed cell death protein 1 (PD-L1) monoclonal antibody. The combination was found to have a favorable toxicity profile, a 53% objective response rate (21/40 patients), and a median duration of response of 10.3 months in advanced HER2 positive /PD-L1 positive GEA and was being investigated as a possible first-line therapy for GEA. This study was discontinued for business reasons.13
A phase II trial combined margetuximab with pablizumab as second-line treatment for HER2 and/or PD-L1 positive patients with advanced GEA, resulting in significant improvement in patient survival and an objective response rate of 28.2% and a median overall survival of 13.9 months.14
Zanidatamab (ZW25)
Zanidatamab is a bispecific monoclonal antibody that binds to two non-overlapping extracellular regions on HER2 (ECD4 and ECD2), resulting in the formation of HER2 clusters and induction of greater internalization and downregulation of HER2 as compared to trastuzumab as seen in preclinical studies.15 This is similar to trastuzumab and pertuzumab, which individually target the same two extracellular regions.16 Early studies have established zanidatamab to have a manageable safety profile when used both as monotherapy, and in combination with chemotherapy in later-line treatment. As a first-line treatment in the phase II trial, zanidatamab demonstrated an objective response rate of 75%, a median duration of response of 16.4 months, and a median progression-free survival of 12 months.17 The Phase III HERIZON-GEA-01 trial is currently underway.18
Zanidatamab is also being studied as a combination regimen with the PD-1 monoclonal antibody tislelizumab and chemotherapy as part various clinical studies.19-21
KN026
KN026 is an anti-HER2 bispecific antibody binding to HER2 domains II and IV. In an open-label, multicenter phase II trial patients with high-level (N=27), low-level (N=14) HER2-positivity, and no HER2 expression (N=4) received one of three doses of KN026: 10 mg/kg/week, 20 mg/kg/every 2 weeks, or 30 mg/kg/every 3 weeks. The ORR in the high-level HER2 cohort was 56% with a durable response duration of 9.7 months, compared with an ORR of 14% in the low-level HER2 cohort.22 Further studies are required to investigate the use of KN026 in advanced GEA patients with HER2 positivity.
Tyrosine Kinase Inhibitors (TKIs)
Lapatinib
Lapatinib inhibits the activation of MAPK, PI3K-AKT, and phospholipase-Cg signaling pathways by blocking both HER1 and HER2.4 The effectiveness of TKIs in the treatment of GEA is limited. While there have been trials with a combination of lapatinib with chemotherapeutic agents, namely the TyTAN23and LOGiC24 trials, that evaluated a combination of lapatinib with paclitaxel versus paclitaxel alone and a combination of lapatinib with capecitabine plus oxaliplatin, respectively, there was no improvement seen in overall survival rates in patients with HER2-positive GEA. The toxicity of lapatinib as well as patient demographics, including age and region may have had a role to play in the observed lack of improvement seen.
Other TKIs
Other TKIs that have shown some antitumor activity in HER2-positive GEA include afatinib25 and tucatinib26, but further studies are required to determine their efficacy in the treatment of advanced GEA.
Antibody Drug Conjugates (ADCs)
Fam-Trastuzumab deruxtecan-nxki
Fam-trastuzumab deruxtecan-nxki is an antibody-drug conjugate (ADC) consisting of trastuzumab and a cytotoxic topoisomerase I inhibitor connected by a cleavable tetrapeptide-based linker. This molecule, with its capacity to diffuse through the cell membrane of targeted cells, can also target surrounding non-HER2-positive cells, a phenomenon known as the ‘bystander effect’.9 The DESTINY-Gastric01 trial was a phase II study that included 187 Asian patients with advanced disease following at least two prior therapies including trastuzumab. Patients were randomized 2:1 to receive the ADC (6.4 mg/kg q21 days) or paclitaxel/irinotecan monotherapy. The objective response rate (ORR) for patients on the ADC was 51% compared with 14% for patients on the chemotherapeutic regimen (complete responses were 9% versus 0%, P<.001), with a median PFS of 5.6 months versus 3.5 months. Responses were proportional to the HER2 expression status, with 58% in HER2 3+ versus 29% in the HER2 2+/FISH+ subgroups.27
In Western patients, a phase II non-randomized DESTINY-Gastric02 trial conducted with 79 patients with HER2 positive metastatic GEA who progressed to trastuzumab, received fam-trastuzumab deruxtecan-nxki as second-line treatment. Recent results show an ORR of 41.8%, a median OS of 12.1 months, and a median PFS of 5.6 months. The most frequent adverse events included nausea, vomiting, and fatigue, and interstitial lung disease (10.1%).28
There are ongoing trials studying the safety and efficacy of fam-trastuzumab deruxtecan-nxki in combination with chemotherapy in GEA (DESTINY-Gastric03, NCT04379596).29
Other ADCs
RC48
A phase II single-arm study of the safety and efficacy of RC48 as third-line treatment of 179 HER2-positive patients with locally advanced or metastatic GEA who were under at least one second-line treatment has shown positive results with an ORR of 25%, and a median OS of 7.6 months.30
ARX788
The ACE-Gastric-01 phase I study has shown good tolerability and antitumor activity of ARX788, a molecule with zanidatamab-specific double antibody components and ADC drugs, the treatment of HER2-positive patients with advanced gastric cancer and GEA.31
Approved and Investigational Treatments for BTC
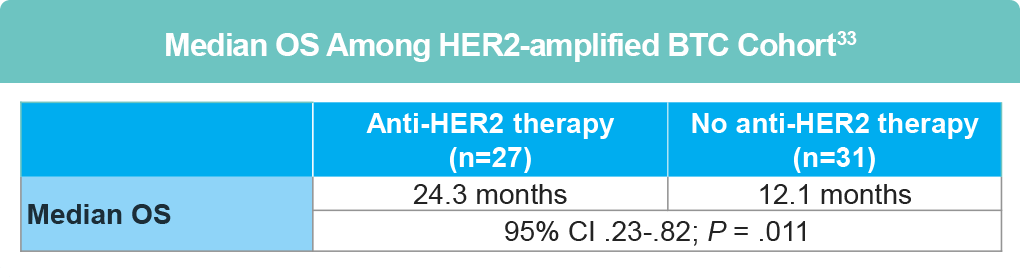
Several clinical trials assessing combination chemotherapy + anti-EGFR targeted therapy for advanced BTC have been conducted, with varying results in survival benefit. However, small studies investigating treatment of metastatic HER2-amplified or HER2-mutated GBC with HER2-targeted treatments (including trastuzumab and neratinib) have been successful.32 The Japanese GOZILA and MONSTAR studies and American COLOMATE study demonstrated that of those with HER-amplified BTC, significant benefit was derived for those receiving anti-HER2 directed therapy, with significantly longer overall survival (OS) compared to those not receiving targeted therapy.33
Antibodies
Zanidatamab (ZW25)
Zanidatamab is now the first FDA-approved bispecific antibody targeting HER2 in adults with previously treated unresectable or metastatic HER2-positive (IHC 3+) biliary tract cancer (BTC).
Zanidatamab, the first HER2 targeted therapy to be approved by the FDA for the treatment of BTC in adults with previously treated, unresectable or metastatic HER2 positive (IHC 3+) BTC as detected by an FDA-approved test.35,36
This approval was based on results from the HERIZON-BTC-01 trial, an open-label multicenter, single arm trial in 62 patients with unresectable or metastatic HER2 positive (IHC 3+ by an FDA-approved test) BTC.34 While all enrolled patients had previously received at least 1 gemcitabine-containing regimen in the advanced disease setting, 31% of participants had received 2 prior lines of therapy, and 10% had received ≥ 3 lines of therapy.35
Of the 62 participants, 52% achieved an ORR (3% CR, 48% PR), with a median DoR of 14.9 months.35 Common adverse events (mostly low-grade) included diarrhea, infusion reactions, abdominal pain and fatigue.35
Additionally, a phase-III study, HERIZON-BTC-302 (NCT06282575), is currently underway to evaluate the combination of zanidatamab + standard of care (SOC) therapy vs SOC monotherapy in the first-line setting for HER2+ BTC.36,37
Trastuzumab
A phase II clinical trial demonstrated an ORR of 66.6% with trastuzumab for the first- or second-line treatment of HER2-amplified gallbladder cancer.4 The MyPathway phase IIa trial showed that a combination of trastuzumab and pertuzumab improved efficacy in patients with HER2 positive BTC, with an ORR of 23%.37
A prospective pilot study of a trastuzumab biosimilar (trastuzumab-pkrb) in combination with chemotherapy (gemcitabine plus cisplatin) showed promising preliminary efficacy/safety results when used as a fist-line treatment in 4 HER2 positive patients with advanced BTC.38
A single-arm phase II study evaluated a combination of trastuzumab with FOLFOX regimen in 34 patients with HER2 amplification/overexpression with BTC who were refractory to gemcitabine plus cisplatin combination therapy were. Results were promising with an ORR of 29.4%, a disease control rate of 79.4%, and a median OS of 10.7 months.39
TKIs
Lapatinib
Lapatinib has not shown any efficacy for the treatment of BTC.4
Neratinib
Neratinib is being investigated as a second-line treatment for HER2 mutated BT and preliminary results have shown an ORR of 16%, a median PFS of 2.8 months, and a median OS of 5.4 months. Further research is required to validate these results.40
ADCs
Fam-Trastuzumab deruxtecan nxki (T-DXd)
Patients with advanced BTC who were either HER2-positive (IHC3+ or IHC2+/ISH+, N=24)) or HER2-low-expressing (IHC/ISH of 0/+, 1+/-, 1+/+, or 2+/-, N=8), and intolerant or refractory to gemcitabine containing regimen received 5.4 mg/kg of T-DXd every 3 weeks in the phase II HERB study. There was a statistically significant ORR in HER2 positive patients of 36.4%, with a median OS of 7.1 months. Efficacy results in the HER2-low-expressing group were also encouraging with an ORR of 12.5% and median OS of 8.9 months. The safety profile included an occurrence of interstitial disease, which is consistent with other T-DXd studies and needs careful monitoring.41,42
An open-label phase II study also evaluated the efficacy of T-DXd in HER2-expressing (IHC 3+/2+) cancers (7 types including a BTC cohort) after one or more prior systemic treatments or without alternative treatments. Taken together, the ORR was 37.1% in all 267 patients with responses seen in all cohorts. The greatest benefit was observed in patients with IHC3+ (ORR of 53.4% versus 22% in all patients with BTC), supporting the role of T-DXd for the treatment of solid tumors that overexpress HER2.43
As of April 2024, T-Dxd is FDA-approved for the treatment of adult patients with unresectable or metastatic HER2-positive (IHC 3+) solid tumors who have received prior systemic treatment and have no satisfactory alternative treatment options.44
RC48
A clinical trial studying the efficacy of RC48 in HER2+ unresectable BTCs is currently underway (NCT04329429).45
References
- Lau-Min KS, Li Y, Eads JR, Mamtani R, Getz KD. Association between timely targeted treatment and outcomes in patients with metastatic HER2-overexpressing gastroesophageal adenocarcinoma. Cancer. 2022;128:1853-1862. doi:10.1002/cncr.34117
- Pietrantonio F, Caporale M, Scartozzi M, et al. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: Implication for further clinical research. J. Cancer. 2016;139:2859-2864. doi:10.1002/ijc.30408
- Catenacci DVT, Moy a, Lomnicki A, et al. Personalized antibodies for gastroesophageal adenocarcinoma (PANGEA): A Phase II study evaluating an individualized treatment strategy for metastatic disease. Cancer Discov. 2021;11:308-325.
- Zhu K, Yang X, Tai H, Zhong X, Luo T, Zheng H. HER2-targeted therapies in cancer: a systematic review. Biomarker Res. 2024;12:16. doi:10.1186/s40364-024-00565-1
- Stroes CI, van den Ende T, Derks S, van Laarhoven HWM. A systematic review of HER2 blockade for the curative treatment of gastroesophageal adenocarcinoma: Successes achieved and opportunities ahead. Anti-tumor Treatment. 2021;99:102249. doi:10.1016/j.ctrv.2021.102249
- Bang YJ, van Custem E, Feyereislove A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. 2010;376:687-697. doi:10.1016/S0140-6736(10)61121-X
- Zhao D, Klempner SJ. Chao J. Progress and challenges in HER2-positive gastroesophageal adenocarcinoma. J Hematol Oncol. 2019;12:50. doi:10.1186/s13045-019-0737-2
- Janjigian YY, Kawazoe A, Bai Y, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: Interim analyses from the phase 3 KEYNOTE-811 randomized placebo-controlled trial. Lancet. 2023;402:2197-2208.
- Petrillo A, Smyth EC, van Laarhoven HWM. Emerging targets in gastroesophageal adenocarcinoma: What the future looks like. Ther Adv Med Oncol. 2023;15:17588359231173177. doi:10.1177/17588359231173177
- Rha S, Kim CG, Jung M, et al. Multicenter phase 1b/II study of second-line trastuzumab, ramucirumab, and paclitaxel in patients with HER2-positive advanced gastric or gastroesophageal junction cancer. Updated HER-RAM study with biomarker analysis. J Clin Oncol. 2022;40:330.
- Swain SM, Miles D, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461-471. doi:10.1016/S1470-2045(13)70130-X
- Wagner AD, Grabsh HI, Mauer M, et al. EORTC-1203-GITCG – the “INNOVATION”- trial: Effect of chemotherapy alone versus chemotherapy plus trastuzumab, versus chemotherapy plus trastuzumab plus pertuzumab, in the perioperative treatment of HER2 positive, gastric, and gastroesophageal junction adenocarcinoma on pathologic response rate: a randomized phase II-intergroup trial of the EORTC-Gastrointestinal Tract Cancer Group, Korean Cancer Study Group and Dutch Upper GI-Cancer group. BMC Cancer. 2019;19:494.
- Catenacci DVT, Kang YK, Shim By, et al. Margetuximab with retifanlimab as first-line therapy in HER2+/PD-L1+ unresectable or metastatic gastroesophageal adenocarcinoma: MAHOGANY cohort A. ESMO Open. 2022;7:100563. doi:10.1016/j.esmoop.2022.100563
- Catenacci DVT, Kang YK, Park H, et al. Margetuximab plus pembrolizumab in patients with previously treated HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): A single-arm, phase 1b-2 trial. Lancet Oncol. 2020;21:1066-1076. doi:10.1016/S1470-2045(20)30326-0
- Tabernero J, Shen L, Elimova E, et al. HERIZON-GEA-01: Zanidatamab + chemo ± tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future Oncol. 2022;18:3255-3266. doi:10.2217/fon-2022-0595
- Ayasun R, Ozer M, Sahin I. The role of HER2 status in biliary tract cancers. Cancers. 2023;15:2628. doi:10.3390/cancers15092628
- Ku G, Elimova E, Denlinger CS, et al. 1380P Phase (Ph) II2 study of zanidatamab + chemotherapy (chemo) in first-line (1L) HER2 expressing gastroesophageal adenocarcinoma (GEA). Ann Oncol. 2021;32(suppl 5):S1044-S1045. doi:10.1016/j.annonc.2021.08.1489
- A study of zanidatamab in combination with chemotherapy plus or minus tislelizumab in patients with HER2-positive advanced or metastatic gastric and esophageal cancers (HERIZON-GEA-01). https://clinicaltrials.gov/study/NCT05152147
- Anti-HER2 bispecific antibody ZW25 activity in combination with chemotherapy with/without tislelizumab. https://clinicaltrials.gov/study/NCT04276493
- Study to evaluate the safety and efficacy of tislelizumab in combination with zanidatamab as a 2nd line in HER2-positive advanced gastric cancer in K-Umbrella trial. https://clinicaltrials.gov/study/NCT05270889
- A safety and efficacy study of ZW25 (zanidatamab) plus combination chemotherapy in HER2 expression gastrointestinal cancers, including gastroesophageal adenocarcinoma, biliary tract cancer, and colorectal cancer. https://clinicaltrials.gov/study/NCT03929666
- Xu J, et al. KN026 (anti-HER2 bispecific antibody) in patients with previously treated, advanced HER2-expressing gastric or gastroesophageal junction cancer. Eur J Cancer. 2023;178:P1-12. doi:10.1016/j.ejca.2022.10.004
- Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN—a randomized, phase III study. J Clin Oncol. 2014;32:2039-2049. doi:10.1200/JCO.2013.53.6136
- Hecht JR, Bang YJ. Qin SK, et al. Lapatinib in combination with capecitabine plus oxaliplatin in human epidermal growth factor receptor 2-positive advanced or metastatic gastric, esophageal, or gastroesophageal adenocarcinoma: TRIO-013/LOGiC–a randomized phase III trial. J Clin Oncol. 2016;34:443-451. doi:10.1200/JCO.2015.62.6598
- Janjigian YY, Ku GY, Ilson DH, et al. A phase II study of afatinib in patients (pts) with metastatic human epidermal growth factor receptor (HER2)-positive trastuzumab refractory esophagogastric (EG) cancer. J Clin Oncol. 2015;33(3_suppl):59. doi:10.1200/jco.2015.33.3_suppl.59
- Catenacci DV, Strickler JH, Shitara K, et al. 1434TiP MOUNTAINEER-02: Phase II/III study of tucatinib, trastuzumab, ramucirumab, and paclitaxel in previously treated HER2+ gastric or gastroesophageal junction adenocarcinoma—Trial in progress. Ann Oncol. 2021;32(suppl 5):S1071-S1072. doi:10.1016/j.annonc.2021.08.1543
- Shitara K, Bang YJ, Iwasa S, et al. DESTINY-Gastric01 Investigators. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N Engl J Med. 2020;382:2419-2430. doi:10.1056/NEJMoa2004413
- Ku GY, Di Bartolomeo M, Smyth E, et al. 1205MO Updated analysis of DESTINY-Gastric02: A phase II single-arm trial of trastuzumab deruxtecan (T-DXd) in western patients (Pts) with HER2-positive (HER2+) unresectable/metastatic gastric/gastroesophageal junction (GEJ) cancer who progressed on or after trastuzumab-containing regimen. Ann Oncol. 2022;33(suppl 7):S1100. doi:10.1016/j.annonc.2022.07.1323
- Janjigian YY, Oh DY, Rha SY, et al. Dose-escalation and dose-expansion study of trastuzumab deruxtecan (T-DXd) monotherapy and combinations in patients (pts) with advanced/metastatic HER2+ gastric cancer (GC)/gastroesophageal junction adenocarcinoma (GEJA): DESTINY-Gastric03. J Clin Oncol. 2022;40(4_suppl):295. doi:10.1200/JCO.2022.40.4_suppl.295
- Peng Z, Liu T, Wei J, et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: A single-arm phase II study. Cancer Commun (Lond).2021;41:1173-1182. doi:10.1002/cac2.12214
- Hurvitz SA, Park H, Frentzas S, et al. Safety and unique pharmacokinetic profile of ARX788, a site-specific ADC, in heavily pretreated patients with HER2-overexpressing solid tumors: Results from two phase 1 clinical trials. J Clin Oncol. 2021;39(15_suppl):1038. doi:10.1200/JCO.2021.39.15_suppl.1038
- Athauda A, Fong C, Lau DK, et al. Broadening the therapeutic horizon of advanced biliary tract cancer through molecular characterization. Cancer Treat Rev. 2020;86:101998. doi:10.1016/j.ctrv.2020.101998
- Inoue K, Nakamura Y, Caughey B, et al. Comprehensive clinic-molecular profile and efficacy of anti-HER2 therapy for HER2-amplified biliary tract cancer. J Clin Oncol. 2024;42(3-suppl):544. doi:10.1200/JCO.2024.42.3_suppl.544
- News Release. 11/20/24 (https://www.prnewswire.com/news-releases/jazz-pharmaceuticals-announces-us-fda-approval-of-ziihera-zanidatamab-hrii-for-the-treatment-of-adults-with-previously-treated-unresectable-or-metastatic-her2-positive-ihc-3-biliary-tract-cancer-btc-302312216.html).
- Zanidatamab (Ziihera®) Prescribing Information (PI) 11/2024 (https://pp.jazzpharma.com/pi/ziihera.en.USPI.pdf).
- Ernst D. Ziihera approved for HER2-positive biliary tract cancer. Medical Professionals Reference (MPR). Published 11/21/24 (https://www.empr.com/news/ziihera-approved-for-her2-positive-biliary-tract-cancer/).
- Pant S, Ducreux M, Harding JJ, et al. A phase IIb, open-label, single-arm study of zanidatamab (ZW25) monotherapy in subjects with advanced or metastatic HER2-amplified biliary tract cancers. J Clin Oncol. 2021;39(3_suppl):TPS352. doi:10.1200/JCO.2021.39.3_suppl.TPS352
- Javle M, Borad MJ, Azad NS, et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021;22:1290-1300. doi:10.1016/S1470-2045(21)00336-3
- Jeong H, Jeong JH, Kim SP, et al. Feasibility of HER2-targeted therapy in advanced biliary tract cancer: A prospective pilot sudy of trastuzumab biosimilar in combination with gemcitabine plus cisplatin. Cancers (Basel). 2021;13:161. doi:10.3390/cancers13020161
- Lee CK, Chon HJ, Cheon J, et al. Trastuzumab plus FOLFOX for HER2-positive biliary tract cancer refractory to gemcitabine and cisplatin: A multi-institutional phase 2 trial of the Korean Cancer Study Group (KCSG-HB19-14). Lancet Gastroenterol Hepatol. 2023;8:56-65. doi:10.1016/S2468-1253(22)00335-1
- Tao Z, Xiangyu A, Shen K, Zhao Y, Zeng H, Ma X. Safety and efficacy profile of neratinib: A systematic review and meta-analysis of 23 prospective clinical trials. Clin Drug Investig. 2019;39:27-43. doi:10.1007/s40261-018-0719-0
- Ohba A, Morizane C, Kawamoto Y, et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): An investigator-initiated multicenter phase 2 study (HERB trial). J Clin Oncol. 2022;40(16_suppl):4006. doi:10.1200/JCO.2022.40.16_suppl.4006
- Meric-Bernstam F, Makker V, Oaknin A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: Primary results from the DESTINY-PanTumor02 phase II trial. J Clin Oncol 2024;42:47-58. doi:10.1200/JCO.23.02005
- Daiichi Sankyo: Press release. ENHERTU® approved in the US as first tumor agnostic HER2 directed therapy for previously treated patients with metastatic HER2 positive solid tumors. April 5, 2024. https://www.daiichisankyo.com/files/news/pressrelease/pdf/202404/20240405_E.pdf
- A study of RC48-ADC in subjects with HER2 overexpressed metastatic biliary tract cancer. https://clinicaltrials.gov/study/NCT04329429
All URLs accessed April 12, 2025