HER2+ Guidelines for Diagnosis and Treatment of GEA and BTC
A multidisciplinary approach via collaboration between oncologists and pathologists is recommended for effective diagnosis and treatment of GEA and BTC.
Diagnosis of GEA
The 2016 HER2 testing and clinical decision making guidelines in GEA from the College of American Pathologists (CAP), American Society for Clinical Pathology (ASCP), and American Society of Clinical Oncology (ASCO) and the most recently updated National Comprehensive Cancer Network (NCCN) guidelines for Gastric Esophageal and Esophagogastric Junction Cancers recommend assessment of overexpression or amplification of HER2 for patients with inoperable, locally advanced, recurrent, or metastatic adenocarcinomas being considered for treatment with trastuzumab.1,2
Assessment for tumor HER2 overexpression should be done using immunohistochemistry (IHC) first, followed by fluorescence in situ hybridization (FISH) or other in situ hybridization (ISH) techniques when there is 2+ equivocal expression by IHC.2 In cases with positive (3+) or negative (0 or 1+) HER2 IHC, further ISH testing is not recommended.2
Additionally, subclassification of GEA as intestinal or diffuse type may impact treatment approach, as intestinal type tumors are more likely to overexpress HER2.3 Tissue selection by pathology for assessment should come from areas of lowest grade tumor, or those with intestinal morphology. This recommendation stems from these areas being more likely to demonstrate HER2-positive results.2
Guidelines recommend repeat biomarker testing when there is clinical or radiologic progression. NGS, which can assess numerous mutations simultaneously, is to be considered for single biomarkers, such as HER2 only when limited diagnostic tissue is available, if the patient is unable to undergo a traditional biopsy, or at the discretion of the treating physician.1 The Hofmann method for scoring HER2 IHC and ISH results for GEA is recommended (click here for HER2 scoring).4
Diagnosis of BTC
Diagnosis of differing subtypes of BTC can be challenging. For instance, with gallbladder cancer (GBC), diagnosis is often incidental at cholecystectomy and typically of advanced stage, due to lack of symptoms or symptoms that mimic biliary colic/chronic cholecystitis. Guideline recommendations for initial workup of GBC includes liver function testing, computed tomography (CT) and/or magnetic resonance imaging (MRI) of the chest, abdomen, and pelvis for assessment of tumor penetration through the gallbladder wall as well as nodal/distant metastasis. CT can be particularly useful for detection of lymph node involvement, while MRI may be helpful in distinguishing GBC from benign disease. The presence of jaundice with GBC is associated with poor prognosis. For cholangiocarcinoma (CCA), the optimal diagnostic approach is core needle biopsy.5
The NCCN guidelines recommend molecular testing for HER2 overexpression and/or amplification in unresectable or metastatic BTCs, including gallbladder, intrahepatic CCA, and extrahepatic CCA, due to the significant incidence of HER2 overexpression and/or amplification in CCAs and gallbladder cancer. As is the case with GEAs, HER2 amplification can be detected by IHC, FISH, or NGS techniques, with NGS being considered upfront in the case of limited availability of diagnostic tissue.5
Guidelines in the treatment of advanced GEA
Chemotherapy with oxaliplatin preferred over cisplatin in systemic therapy for unresectable locally advanced, recurrent, or metastatic disease (where local therapy is not indicated). Targeted treatment for HER2-positive GEAs may offer additional therapeutic approaches.1,2
Trastuzumab or an FDA-approved biosimilar must be added to first-line chemotherapy for advanced HER2 overexpression positive GEAs. Other regimens for HER2-positive GEA include:
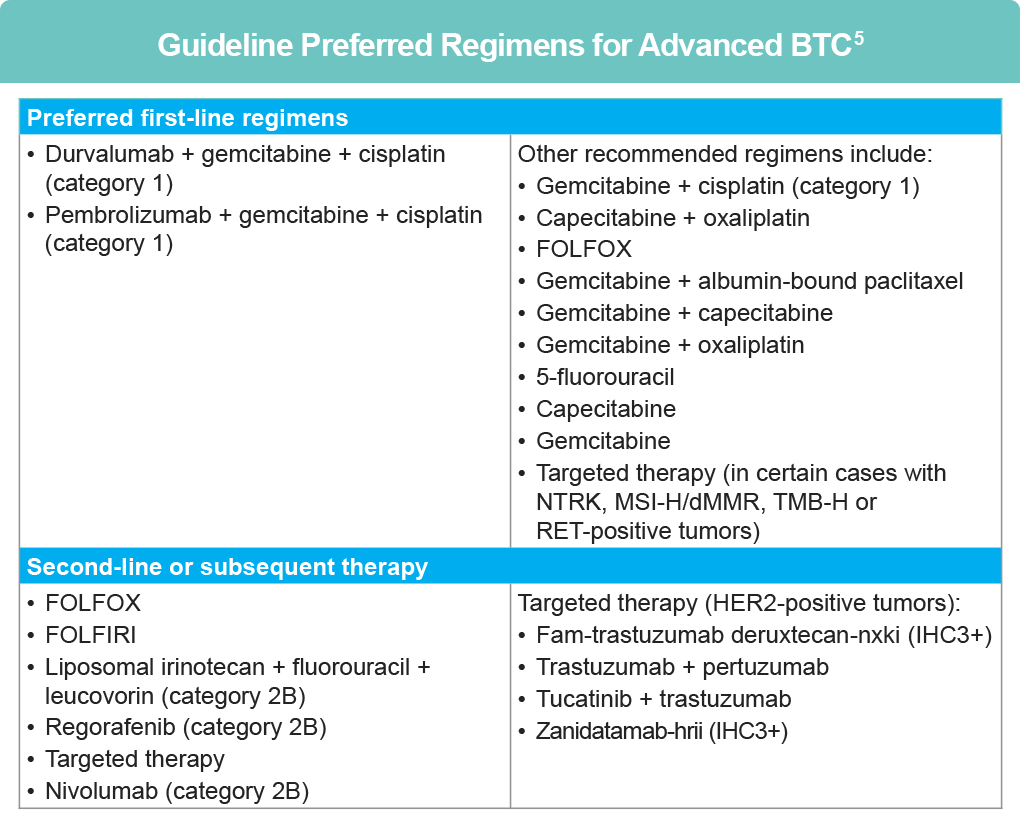
Guidelines in the treatment of advanced BTC
Per the NCCN, small case series and biomarker-selected trials have suggested efficacy of HER2-directed therapies in BTC.5 Currently, first-line treatments for unresectable and metastatic BTC include chemotherapy with PD-1 directed immunotherapy; HER2 directed treatment is included in subsequent therapy regimens in the event of disease progression.5
References
- National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology. Esophageal and Esophagogastric Junction Cancers (Version 2.2025). https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- Bartley AN, Washington MK, Ventura CB, et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma; guideline from the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Arch Pathol Lab Med. 2016;140:1345-1363. doi:10.5858/arpa.2016-0331-CP
- National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology. Gastric Cancer (Version 1.2025). https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- Yaseen A, Bishop J. Assessment of HER2 status in gastroesophageal adenocarcinoma (GEA). UC Davis Health. Cancer Care. June 15, 2019. https://health.ucdavis.edu/blog/lab-best-practice/assessment-of-her2-status-in-gastroesophageal-adenocarcinoma-gea/2019/06
- National Comprehensive Cancer Networ
- k®. NCCN Clinical Practice Guidelines in Oncology. Biliary Tract Cancers (Version 1.2025) https://www.nccn.org/professionals/physician_gls/pdf/btc.pdf
All URLs accessed April 12, 2025