Colorectal Cancer – Treatments
Treatment Guidelines for Metastatic Colorectal Cancer
The emergence of targeted therapies for the treatment of metastatic colorectal cancer (mCRC) has significantly improved survival but has also resulted in a dilemma in identifying the optimal sequence and combination of agents to best improve patient outcomes. Currently there are no established guidelines for optimal sequencing of cytotoxic or targeted agents in mCRC. Fluorouracil (5-FU) is considered the backbone of mCRC treatment. However, all the regimens listed below are generally considered interchangeable.
Table 1: Initial Systemic Therapy for Advanced or Metastatic Colorectal Cancer1
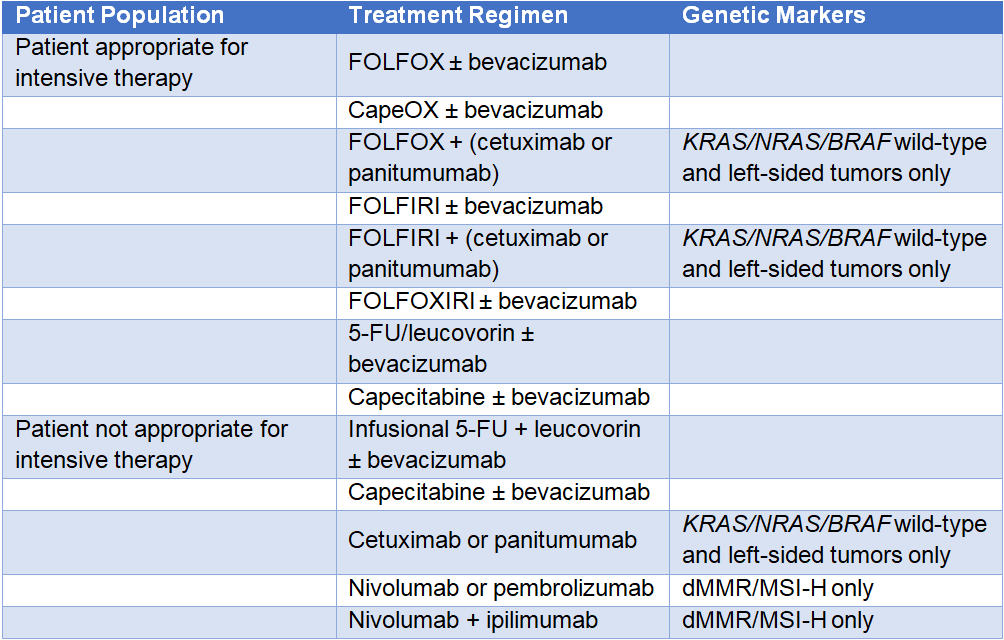
FOLFOX = leucovorin + 5-FU (fluorouracil) + oxaliplatin; CapeOX = capecitabine + oxaliplatin; FOLFIRI = leucovorin + 5-FU + irinotecan;
FOLFOXIRI = leucovorin + 5-FU + oxaliplatin + irinotecan; dMMR = mismatch repair deficient; MSI-H = microsatellite instability-high
Table 2 presents options for subsequent therapy following mCRC disease progression. The choice of second-line or subsequent therapy depends on previously used chemotherapy agents.
Table 2: Subsequent Systemic Therapy for Advanced or Metastatic Colorectal Cancer 1
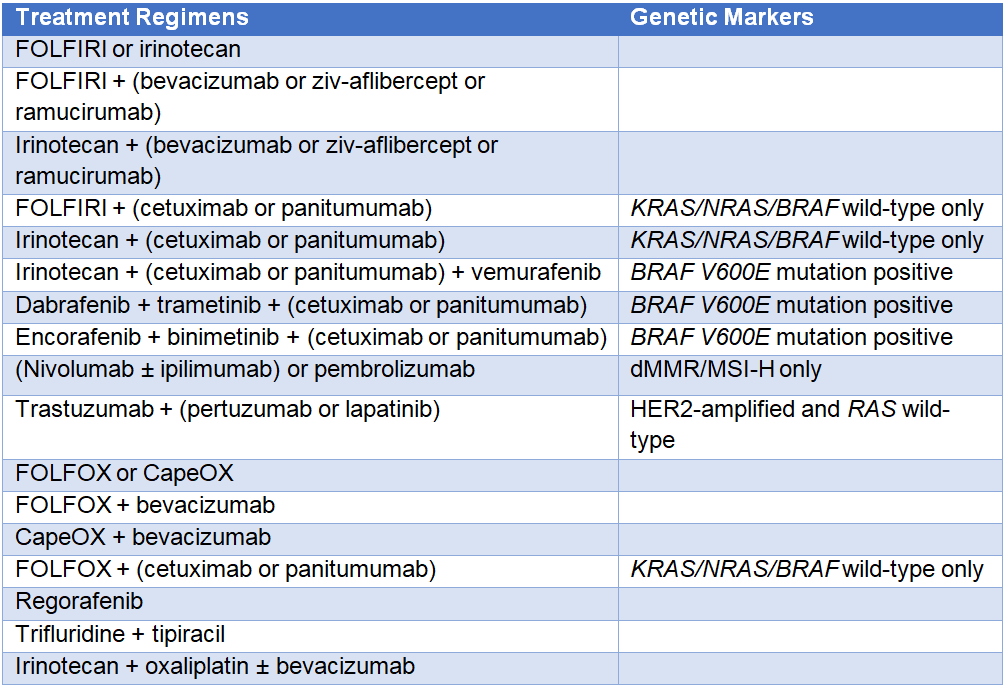
FOLFIRI = leucovorin + 5-FU (fluorouracil) + irinotecan; FOLFOX = leucovorin + 5-FU + oxaliplatin;
CapeOX = capecitabine + oxaliplatin; dMMR = mismatch repair deficient; MSI-H = microsatellite instability-high
Clinical trials
Bevacizumab
Recombinant humanized monoclonal IgG1 antibody that binds to and inhibits the activity of vascular endothelial growth factor (VEGF)2
- The Cancer and Leukemia Group B/Southwest Oncology Group (CALGB/SWOG) 80405 phase 3 trial investigated the efficacy and safety of irinotecan + 5-FU + leucovorin (FOLFIRI) compared with oxaliplatin + 5-FU + leucovorin (mFOLFOX6) with bevacizumab or cetuximab in patients who were previously untreated and were KRAS wild-type. The overall survival (OS) in the bevacizumab and chemotherapy group was 29 months, compared with 29.9 months in the cetuximab and chemotherapy group (hazard ratio [HR] = 0.92; 95% confidence interval [CI], 0.78–1.09; P=0.34). This trial confirmed the safety profile of these two agents as seen in previous trials. The most frequent grade 3 or higher toxicities were hypertension (7%) and gastrointestinal events (2%) for bevacizumab and an acne-like rash (7%) and diarrhea (11%) for cetuximab.3
- In the phase 3 Treatment through Multiple Lines (TML) study, bevacizumab in combination with standard chemotherapy was evaluated as second-line therapy in patients who progressed following a standard bevacizumab-containing regimen in the first-line setting. Continuation of bevacizumab treatment was associated with an increase in median OS of 11.2 months vs 8 months (HR = 0.81; P=0.0062) and in median progression-free survival (PFS) of 5.7 months vs 4.1 months (HR = 0.68; P<0.0001) versus chemotherapy alone. Grade 3–5 bleeding or hemorrhage and venous thromboembolisms were more common in the bevacizumab plus chemotherapy group than in the chemotherapy alone group.2
Ramucirumab
Fully human immunoglobulin G1 (IgG1) monoclonal antibody that binds to VEGF receptor-2 (VEGFR-2), preventing VEGF ligand binding and the formation of new blood vessels4
- In a phase 2 trial, 48 treatment-naïve patients with mCRC received ramucirumab with mFOLFOX6 as first-line therapy. Combination therapy with ramucirumab was efficacious in this patient population, with a median PFS of 5 months, a median OS of 20.4 months, and an objective response rate (ORR) of 58%. This drug combination was also reasonably well tolerated, and the most frequently observed ramucirumab-related grade ≥3 adverse events were hypertension and proteinuria.4
- The efficacy and safety of ramucirumab versus placebo in combination with second-line FOLFIRI for mCRC in 1072 patients with disease progression during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine was assessed in the phase 3 RAISE trial. Median OS was 13.3 months for patients in the ramucirumab group versus 11.7 months for the placebo group (HR = 0.844; 95% CI, 0.730–976; log-rank P=0.0219). The survival benefit was consistent across subgroups of patients who received ramucirumab plus FOLFIRI. The study authors concluded that ramucirumab plus FOLFIRI significantly improved OS compared with placebo plus FOLFIRI as second-line treatment for patients with mCRC. No unexpected adverse events were identified, and toxic effects were judged to be manageable.5
Ziv-aflibercept
Recombinant fusion protein that binds to VEGF and placental growth factor (PlGF), preventing the activation of VEGFR-1 and -26
- A phase 2 trial found that the addition of aflibercept to 74 bevacizumab-naïve or bevacizumab-treated patients resulted in a similar PFS of 0 months vs 2.4 months, respectively, and OS of 10.4 vs 8.5 months, respectively.6
- In the phase 3 VELOUR trial, aflibercept plus FOLFIRI was evaluated in 1226 patients with mCRC previously treated with an oxaliplatin-containing regimen. In this study, the ORR was 19.8% in the aflibercept arm compared with 11.1% in the placebo arm (P=0.0001), PFS was 9.0 vs 4.67 months, respectively (HR = 0.758; P<0.0001), and OS was 13.5 vs 12.06 months, respectively (HR = 0.817; P=0.0032). Although the study was not powered to show a treatment difference between arms, a pre-planned subgroup analysis showed that prior bevacizumab use did not influence aflibercept’s effect on PFS and OS. Toxicities related to aflibercept were comparable with bevacizumab. The VELOUR trial has established the efficacy of aflibercept in a mCRC patient population, approximately one-third of whom had progressed on a prior regimen containing bevacizumab.7
Cetuximab
Recombinant chimeric monoclonal antibody that binds to the epidermal growth-factor receptor (EGFR)8
- In the FIRE-3 trial, 592 patients with KRASexon 2 wild-type tumors were randomly assigned to FOLFIRI plus cetuximab or FOLFIRI plus bevacizumab. Although the proportion of patients who achieved an objective response did not significantly differ between the FOLFIRI plus cetuximab and the FOLFIRI plus bevacizumab groups, FOLFIRI plus cetuximab was associated with longer OS (P=0.017).8
- Patients with KRAS wild-type mutations from the FIRE-3 trial were also assessed in a secondary analysis by subsequent line of therapy. Patients who started cetuximab versus bevacizumab during first-line therapy demonstrated a significantly longer PFS and OS from the start of second-line therapy.9
- In the BOND trial, the efficacy of cetuximab in 329 patients who were refractory to irinotecan-based and possibly oxaliplatin-based chemotherapy was examined. Compared with cetuximab monotherapy, combination with irinotecan resulted in a significantly higher response rate (23% versus 11%; P=0.007) and median time to progression (4.1 months versus 1.5 months; P<0.001).10
Panitumumab
Fully human monoclonal antibody against human EGFR11
- Results from the phase 3 PRIME study of panitumumab as first-line therapy in 1183 previously untreated mCRC patients showed that the combination of panitumumab and FOLFOX4 (leucovorin + 5-FU + oxaliplatin) was well tolerated and significantly improved PFS in KRASwild-type patients compared with chemotherapy alone (10 months versus 8.6 months, respectively; HR = 0.80; P=0.01).11
- The phase 3 trial ASPECCT trial compared panitumumab vs cetuximab as monotherapy for the treatment of mCRC in 999 chemotherapy-refractory, KRASwild-type patients. The study demonstrated that panitumumab is non-inferior to cetuximab, with similar OS benefit in this patient population.12
Pembrolizumab
Monoclonal antibody against PD (programmed death)-1, which blocks the binding of PD-1 on T cells to its ligand PD-L1,13 thereby reactivating an immune system response against tumor cells
- A phase 2 clinical trial of pembrolizumab in 41 patients with progressive metastatic disease demonstrated a notable difference in ORR (50% vs 0%) between those with mismatch repair-deficient (dMMR) or mismatch repair-proficient (pMMR) CRC. Although median PFS and OS have not yet been reached for dMMR patients, the median PFS was 2.2 months, and OS was 5.0 months (HR for disease progression or death = 0.10; P<0.001; HR for death = 0.22; P=0.05) for pMMR patients.13
- The efficacy of pembrolizumab against dMMR/microsatellite instability-high (MSI-H) tumors was demonstrated across multiple tumor types in five single-arm clinical trials, which included patients with CRC. Across these studies, treatment of dMMR/MSI-H tumors with pembrolizumab was associated with an ORR of 39.6%, including complete responses in 7.4% of patients. This included an ORR of 36% in patients with CRC and 46% in other tumor types. Responses lasted for greater than six months in 78% of responding patients.14
- In a study of 86 patients with 12 different cancers who had evidence of dMMR, treatment with pembrolizumab was associated with an ORR of 53%, including a 21% complete response rate.15 On the strength of these results, the indication for pembrolizumab was extended to include previously treated patients with high levels of MSI, regardless of tumor type.
Nivolumab
Anti-PD-1 monoclonal antibody that blocks the binding of PD-1 on T cells to its ligand PD-L1,16 thereby reactivating an immune-system response against tumor cells
- As part of the phase 2 CheckMate-142 study, nivolumab monotherapy was evaluated in a cohort of patients with dMMR, or MSI-H recurrent, or metastatic CRC who had progressed on or after at least one previous line of treatment, including a fluoropyrimidine and oxaliplatin or irinotecan. This trial demonstrated an ORR and disease control rate (DCR) for ≥12 weeks of 31% and 69%, respectively. Patients with BRAF-mutant tumors receiving nivolumab had an investigator-assessed ORR of 25%, which is higher than those reported with chemotherapy or combination treatment including BRAF, EGFR, or MEK inhibitors.17
- In the nivolumab plus ipilimumab (monoclonal antibody targeting cytotoxic T-lymphocyte–associated protein 4) cohort of the CheckMate-142 study, the ORR was 55% and the DCR was 80%. It is interesting to note that responses were observed in patients across all subgroups, including patients with tumors that were positive (≥1%) or negative (<1%) for tumor PD-L1 expression, tumors harboring BRAFor KRAS mutations, and those with and without a clinical history of Lynch syndrome. The patients with BRAF-mutant tumors who received nivolumab plus ipilimumab had an ORR of 55% and a DCR of 79%.16
Regorafenib
Oral multikinase inhibitor that blocks the activity of RET, VEGFR1-3, PDGFR, BRAF, KIT, and RAF-118
- In the phase 3 CORRECT trial, regorafenib was investigated versus best supportive care in mCRC patients who had progressed during or within 3 months of the last standard therapy. Median OS was 6.4 months in the regorafenib group versus 5.0 months in the placebo group (HR = 0.77; P=0.0052). The most common treatment-related grade 3 or worse adverse events were hand-foot skin reaction (17%), fatigue (10%), diarrhea (7%), hypertension (7%), and rash or skin desquamation (6%). These adverse events were mostly manageable with dose reduction or interruption.18
- The phase 3 CONCUR trial has demonstrated that regorafenib significantly improves OS and PFS in an Asian population of patients with previously treated mCRC with no substantial differences in health-related quality of life (HRQoL) versus placebo.19,20
Dabrafenib plus trametinib
A selective BRAF inhibitor combined with a selective MEK inhibitor
- A group of 43 patients with BRAF V600-mutant mCRC were treated with dabrafenib plus trametinib. Of these patients, 12% achieved a partial response or better, including one patient with a complete response, and the duration of the response exceeded 36 months. Stable disease was achieved in 56% of patients.21
Encorafenib plus binimetinib
A selective BRAF inhibitor combined with a selective MEK inhibitor
- In the phase 3 BEACON trial, encorafenib plus binimetinib and cetuximab led to a 48% reduction in the risk of death compared with cetuximab and irinotecan-containing regimens in patients with BRAF V600E-mutant mCRC who previously received up to two lines of therapy. The triplet combination therapy was associated with a median OS of 9.0 months compared with 5.4 months for those who received cetuximab and irinotecan-containing regimens (HR = 0.52; 95% CI, 0.39–0.70; P<0.001), and the ORR was 26.1% vs 1.9%, respectively (P<0.001).22
References
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology: Colon Version 2.2019. nccn.org/professionals/physician_gls/pdf/colon.pdf
- Bennouna J, Sastre J, Arnold D, et al; ML18147 study investigators. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29-37.
- Venook AP, Niedzwiecki D, Lenz H-J, et al. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol. 2014;32(5 suppl): abstract LBA3.
- Garcia-Carbonero R, Rivera F, Maurel J, et al. An open-label phase II study evaluating the safety and efficacy of ramucirumab combined with mFOLFOX-6 as first-line therapy for metastatic colorectal cancer. Oncologist. 2014;19:350-351.
- Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499-508.
- Tang PA, Cohen SJ, Kollmannsberger C, et al. Phase II clinical and pharmacokinetic study of aflibercept in patients with previously treated metastatic colorectal cancer. Clin Cancer Res. 2012;18:6023-6031.
- Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499-3506.
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075.
- Modest DP, Stintzing S, von Weikersthal LF, et al. Impact of subsequent therapies on outcome of the FIRE-3/AIO KRK0306 trial: first-line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild-type tumors in metastatic colorectal cancer. J Clin Oncol. 2015;33:3718-3726.
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med.2004;351:337-345.
- Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol.2014;25:1345-1355.
- Price TJ, Peeters M, Kim TW, et al. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15:569-579.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509-2520.
- Food and Drug Administration (FDA). FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. May 23, 2017. gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. 2017;357:409-413.
- Overman MJ, Lonardi S, Wong KY, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol.2018;36:773-779.
- Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182-1191.
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. 2013;381:303-312.
- Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619-629.
- Qin S, Kim TW, Yau TC, et al. Effects of regorafenib therapy on health-related quality of life (HRQoL) in patients with metastatic colorectal cancer (mCRC) in the phase III CONCUR trial. J Clin Oncol. 2015;33(suppl 3): abstract 697.
- Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J Clin Oncol. 2015;33:4023-4031.
- Kopetz S, Grothey A, Van Cutsem E, et al. BEACON CRC: a randomized, 3-arm, phase 3 study of encorafenib and cetuximab with or without binimetinib vs. choice of either irinotecan or FOLFIRI plus cetuximab in BRAF V600E-mutant metastatic colorectal cancer. Ann Oncol. 2019;30 (suppl 4): abstract LBA-006.
