Hepatocellular Carcinoma – Pathophysiology
Pathophysiology
Hepatocellular carcinoma (HCC) primarily arises in a cirrhotic liver, where repeated inflammation and fibrinogenesis predispose the liver to dysplasia and malignant transformation. Viral infections with hepatitis B virus (HBV) and hepatitis C virus (HCV) lead to enhanced hepatocyte turnover as the liver attempts to replace infected cells that have been immunologically attacked.1 There is some evidence to suggest that HCC develops from hepatic stem cells that proliferate in response to chronic regeneration caused by viral injury. The cells in small dysplastic nodules appear to carry markers consistent with stem cells.2 HBV can also cause HCC in the absence of cirrhosis. HBV integrates its deoxyribonucleic acid (DNA) into the host genome, leading to genomic instability and chromosomal rearrangements. HCV uses ribonucleic acid (RNA) to store genetic information and therefore does not integrate into the host genome. HCV-related HCC is found almost exclusively in patients with cirrhosis.3
In the past, HCC generally presented at an advanced stage with right-upper-quadrant pain, weight loss, and signs of decompensated liver disease. However, routine screening of patients with known cirrhosis and α-fetoprotein (AFP) measurements has led to an increase in early detection.
As most liver cancers are observed in association with cirrhosis, it is important to consider the severity of liver disease when selecting a treatment strategy. Child-Pugh scoring determines the severity of liver disease on the basis of serum albumin, bilirubin, prothrombin time, ascites, and encephalopathy.
Child-Pugh Scores
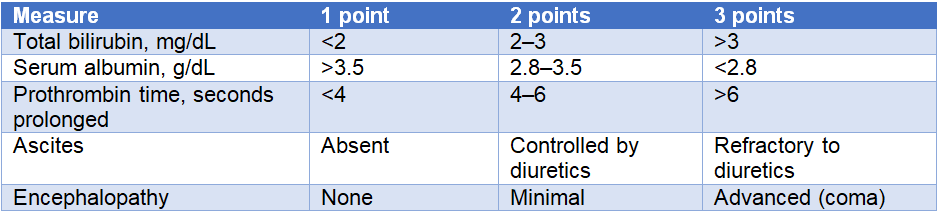
Patients are separated into three classes based on their Child-Pugh scores. Child-Pugh class A has scores of 5–6, class B has scores of 7–9, and class C has scores of 10–15.4
Biomarkers
AFP is a member of the albumin protein family and is produced by the yolk sac and liver during fetal development. AFP levels are high in fetal serum but gradually decrease to adult levels within the first year of life.5 AFP is produced at undetectable or very low levels in healthy adults as the ability to produce it is largely lost in mature hepatocytes. However, transformed liver cancer cells may regain the ability to produce AFP, and levels are usually elevated in patients with HCC. Low concentrations of serum AFP are usually indicative of highly differentiated cancers.6 However, up to 40% of early-stage HCC cases and 15–20% of advanced HCC cases have normal AFP levels.7
AFP is involved in the regulation of cell growth, differentiation, apoptosis, and angiogenesis. Research has suggested that AFP may play a role in HCC carcinogenesis. Importantly, AFP has been implicated in tumor evasion of immune surveillance through the inhibition of immune cells. Dendritic cells act as antigen-presenting cells that process and present antigen to naïve T cells. AFP can inhibit the maturation and induce apoptosis of dendritic cells, thereby preventing activation of T cells directed against the tumor. Patients with HCC exhibiting high levels of AFP have fewer dendritic cells capable of activating T cells in peripheral blood samples.8 Without an adequate number of dendritic cells, the secretion of interleukin-12 (IL-12) in response to the tumor is also decreased. IL-12 is a cytokine that induces the express of activating receptors on natural killer (NK) cells, enhancing the antitumor activity of these immune cells. Although AFP does not directly affect NK cell activity, its inhibition of dendritic cell maturation and the resulting reduction in the secretion of IL-12 indirectly inhibits NK cell function.6
AFP may also play a role in angiogenesis, a process that is essential for the initiation, progression, and metastasis of HCC. Numerous angiogenic factors, such as vascular endothelial growth factor (VEGF), are involved in the regulation of angiogenesis. In HCC, increased serum AFP concentration is correlated with enhanced VEGF-A expression.9 In a study that silenced AFP expression, levels of VEGF and its receptor VEGFR-2 were significantly reduced, and the angiogenic capability of endothelial cells was significantly diminished. AFP secreted by hepatic cancer cells may stimulate the production of VEGF, leading to increased tumor angiogenesis.10
Serum AFP levels increase with disease progression and are highest in metastatic disease. The mechanism for serum AFP elevations during progression is not fully elucidated.11 It has been suggested that elevated serum AFP levels identify a subset of HCC tumors that are larger, associated with poor survival, and present with bi-lobar involvement, portal vein invasion, and poorly differentiated histology.7
Approximately 50% of HCCs secrete AFP, with levels >400 ng/mL considered a reliable marker for supporting a diagnosis of HCC.12 A study by the Surveillance, Epidemiology, and End Results Program (SEER) analyzed the prognostic and predictive role of AFP in 33,820 individuals with HCC. The study found that AFP levels at diagnosis were an independent predictor of pathological grade (odds ratio [OR] = 2.559; 95% confidence interval [CI], 2.075–3.157, P<0.001), TNM-7 stage (OR = 2.794; 95% CI, 2.407–3.242; P<0.001), and tumor size (OR = 1.748; 95% CI, 1.574–1.941; P<0.001). AFP-positive tumors were also more likely than AFP-negative tumors to be poorly differentiated or anaplastic (22.8% vs 10.5%, P<0.001).13
A retrospective study of sorafenib in HCC found worse overall survival (OS) outcomes in patients with AFP levels of 200 ng/ml or higher.14 There are currently no validated references to define high or low AFP concentrations. Most sources cite levels >20 ng/ml as above normal and values >400 ng/ml as high.15 The diagnostic specificity of AFP is very high when levels are above 400 ng/ml. AFP levels >1000 ng/ml have been used to predict the recurrence of HCC in liver transplant patients. Individuals with elevated preoperative AFP levels >1000 ng/ml had 1- and 5-year rates of survival without recurrence of 90% and 52.7%. Levels ≤1000 ng/ml had survival rates of 95% and 80.3% at 1 and 5 years.16
High AFP levels have been associated with elevated VEGFR expression, increased angiogenesis, and poor prognosis in HCC.17,18 An inverse relationship has been observed between response to sorafenib and post-treatment levels of serum AFP.11 Elevated/high AFP (AFP-H, defined as AFP ≥400 ng/mL) was prospectively shown in a phase 3 clinical trial to be predictive for improved OS in patients with advanced HCC who were treated with ramucirumab.19,20 Although the mechanism of this selective benefit is unknown, a considerable proportion of advanced HCC patients are AFP-H, and this group has very poor survival expectations once they progress on first-line sorafenib. Ramucirumab has the potential to fill a gap in the therapeutic armamentarium for a biomarker-selected group of patients with advanced HCC.
References
- Block TM, Mehta AS, Fimmel CJ, Jordan R. Molecular viral oncology of hepatocellular carcinoma. Oncogene. 2003;22:5093-5107.
- Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev. 2005;1:253-260.
- Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(suppl 4):14-22.
- Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42(suppl 1):S100-S107.
- Meng W, Bai B, Bai Z, et al. The immunosuppression role of alpha-fetoprotein in human hepatocellular carcinoma. Discov Med. 2016;21:489-494.
- Wang X, Wang Q. Alpha-fetoprotein and hepatocellular carcinoma immunity. Can J Gastroenterol Hepatol. 2018;2018:9049252.
- Tangkijvanich P, Anukulkarnkusol N, Suwangool P, et al. Clinical characteristics and prognosis of hepatocellular carcinoma: analysis based on serum alpha-fetoprotein levels. J Clin Gastroenterol. 2000;31:302-308.
- Pardee AD, Shi J, Butterfield LH. Tumor-derived α-fetoprotein impairs the differentiation and T cell stimulatory activity of human dendritic cells. J Immunol. 2014;193:5723-5732.
- Corradini SG, Morini S, Liguori F, et al. Differential vascular endothelial growth factor A protein expression between small hepatocellular carcinoma and cirrhosis correlates with serum vascular endothelial growth factor A and alpha-fetoprotein. Liver Int. 2009;29:103-112.
- Meng W, Li X, Bai Z, et al. Silencing alpha-fetoprotein inhibits VEGF and MMP-2/9 production in human hepatocellular carcinoma cell. PLoS One. 2014;9(2):e90660.
- Sauzay C, Petit A, Bourgeois AM, et al. Alpha-foetoprotein (AFP): a multi-purpose marker in hepatocellular carcinoma. Clin Chim Acta.2016;463:39-44.
- Song PP, Xia JF, Inagaki Y, et al. Controversies regarding and perspectives on clinical utility of biomarkers in hepatocellular carcinoma. World J Gastroenterol. 2016;22:262-274.
- Bai DS, Zhang C, Chen P, et al. The prognostic correlation of AFP level at diagnosis with pathological grade, progression, and survival of patients with hepatocellular carcinoma. Sci Rep. 2017;7:12870.
- Bruix J, Cheng AL, Meinhardt G, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol. 2017;67:999-1008.
- Abou-Alfa GK. Ramucirumab and the controversial role of α-fetoprotein in hepatocellular carcinoma. Lancet Oncol. 2019;20:177-179.
- Hameed B, Mehta N, Sapisochin G, et al. Alpha-fetoprotein level >1000 ng/ml as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20:945-951.
- Shan YF, Huang YL, Xie YK, et al. Angiogenesis and clinicopathologic characteristics in different hepatocellular carcinoma subtypes defined by EpCAM and α-fetoprotein expression status. Med Oncol. 2011;28:1012-1016.
- Yamashita T, Forgues M, Wang W, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res.2008;68:1451-1461.
- Zhu AX, Kang YK, Yen CJ, et al. REACH-2: A randomized, double-blind placebo-controlled phase 3 study of ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated baseline alpha-fetoprotein (AFP) following first-line sorafenib. J Clin Oncol. 2018;36(suppl): abstract 4003.
- Zhu A, Finn R, Galle P, et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated alpha-fetoprotein (AFP) following first-line sorafenib: pooled efficacy and safety across two global randomized Phase 3 studies (REACH-2 and REACH). Ann Oncol. 2018;29 (suppl 5): abstract LBA-001.
