Gastric Cancer – Treatments
Treatment Guidelines for Gastric Cancer
Two-drug cytotoxic regimens are preferred for first-line gastric cancer due to the decreased risk of toxicity. Three-drug cytotoxic regimens may be considered for medically fit patients with good performance status and access to frequent evaluations for adverse events.1
For any patients with metastatic adenocarcinoma overexpressing human epidermal growth receptor 2 (HER2), trastuzumab should be added to first-line chemotherapy regimens. Although the preferred regimen is a fluoropyrimidine (fluorouracil or capecitabine) combined with cisplatin, other chemotherapy agents may be considered. Using an anthracycline with trastuzumab is not recommended.1
Table 1. First-line Systemic Therapy for Unresectable Locally Advanced, Recurrent, or Metastatic Disease1
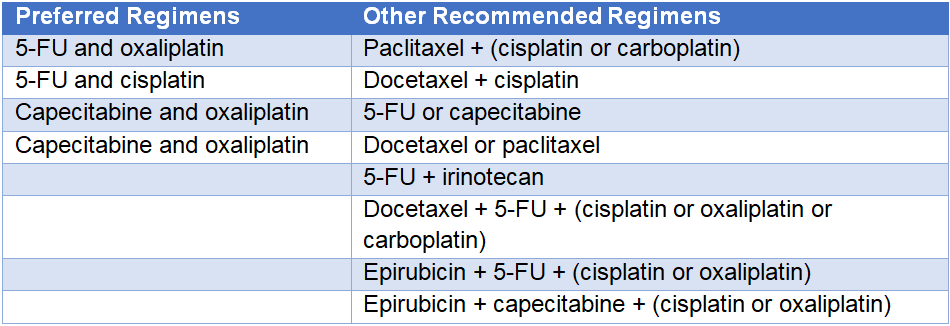
5-FU = fluorouracil.
In spite of the availability of novel therapeutic options, evidence supporting improved outcomes in patients receiving subsequent lines of therapy, and recommendations from evidence-based guidelines, a retrospective analysis of 5221 patients with advanced or metastatic gastric or gastroesophageal-junction (GEJ) cancer indicated that only 64% of patients who received first-line therapy and who were considered a candidate for second-line therapy actually received continued treatment.2
Table 2. Second-line or Subsequent Therapy for Unresectable Locally Advanced, Recurrent, or Metastatic Disease1
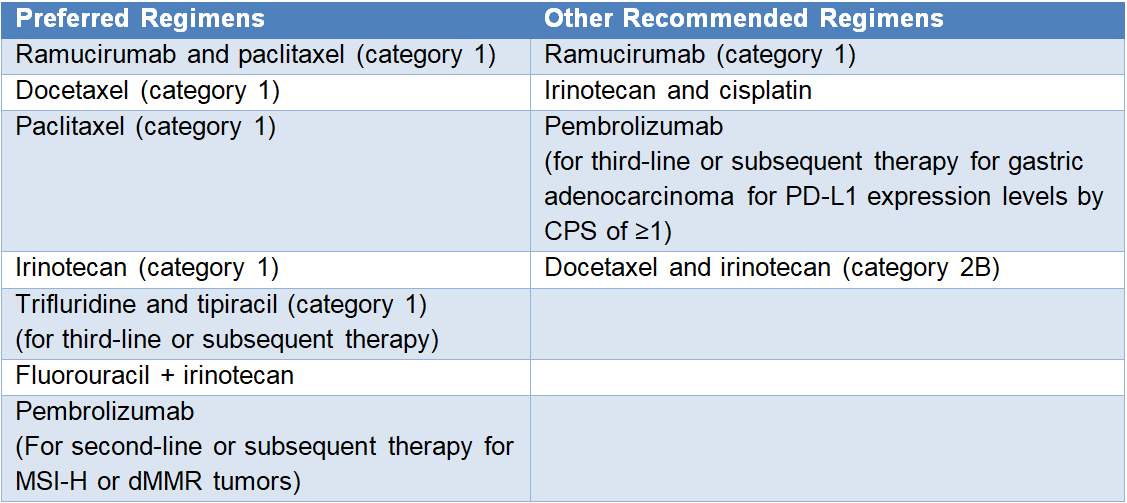
PD-L1 = programmed (cell) death ligand 1; CPS = combined positive score; 5-FU = fluorouracil; MSI-H = microsatellite instability-high; dMMR = mismatch repair-deficient.
Clinical trial data
Despite the availability of novel therapeutic options, the evidence supporting improved outcomes in patients receiving subsequent lines of therapy, and recommendations from evidence-based guidelines, a retrospective analysis of 5221 patients with advanced or metastatic gastric or GEJ cancer indicated that only 64% of those who received first-line therapy and who were considered candidate for second-line actually received continued therapy.2
Ramucirumab
A fully human immunoglobulin G1 (IgG1) monoclonal antibody that binds to vascular endothelial growth factor receptor 2 (VEGFR2), preventing vascular endothelial growth factor (VEGF)-ligand binding and the formation of new blood vessels3
- In the phase 3 RAINBOW trial, 655 patients with advanced gastric or GEJ adenocarcinoma and disease progression after first-line chemotherapy were randomized to receive paclitaxel plus ramucirumab or placebo. Median overall survival (OS) was significantly longer in the ramucirumab plus paclitaxel group than with paclitaxel alone (9.6 months vs 7.4 months; hazard ratio [HR] = 0.807; 95% confidence interval [CI], 0.678–0.962; P=0.017). Ramucirumab also improved median progression-free survival (PFS) by 4.4 months versus 2.9 months (HR = 0.635; P<0.0001) and the objective response rate (ORR) by 28% versus 16% (P<0.0001) compared with paclitaxel alone. Grade 3 or higher adverse events that occurred in the ramucirumab plus paclitaxel group versus paclitaxel alone included neutropenia (41% vs 19%), leukopenia (17% vs 7%), hypertension (14% vs 2%), fatigue (12% vs 5%), anemia (9% vs 10%), abdominal pain (6% vs 3%), and febrile neutropenia (3% vs 2%), respectively.3
- In the phase 3 REGARD trial, 355 patients with advanced gastric or GEJ adenocarcinoma who had progressed after first-line platinum- or fluoropyrimidine-containing chemotherapy were randomized to receive best supportive care plus ramucirumab or placebo. Median OS was significantly longer for the ramucirumab group compared with the placebo group (5.2 months vs 3.8 months; HR = 0.776; 95% CI, 0.603–0.998; P=0.047). Rates of adverse events were similar between the two treatment arms (94% for ramucirumab, 88% for placebo), but the rate of hypertension was higher in the ramucirumab group (16% vs 8%).4
- The phase 3 RAINFALL study randomized 645 patients with metastatic gastric or GEJ adenocarcinoma eligible for first-line chemotherapy and with Eastern Cooperative Oncology Group (ECOG) performance status 0–1 to receive either ramucirumab or placebo. All patients received cisplatin plus capecitabine or fluorouracil (5-FU). RAINFALL met its primary endpoint of improved PFS (median PFS = 5.7 months with ramucirumab vs 5.4 months with placebo; HR = 0.75; P=0.011). However, there was no OS benefit with ramucirumab vs the placebo control (median OS = 11.2 months vs 10.7 months; HR = 0.96; P=0.68).5
Trastuzumab
A recombinant monoclonal antibody that binds to the extracellular domain of HER2 and inhibits the proliferation and survival of HER2-dependent tumors6
- In the phase 3 Trastuzumab for Gastric Cancer (ToGA) trial of 594 patients who had gastric cancer with HER2 overexpression, subjects who were given trastuzumab in addition to cisplatin plus capecitabine or 5-FU had a longer median OS than patients receiving chemotherapy alone (13.8 months vs 11.1 months; HR = 0.74; 95% CI, 0.60–0.91; P=0.0046). Rates of adverse events did not differ between treatment groups. The most common adverse events in each group were nausea (63–67%), vomiting (46–50%), and neutropenia (53–57%). Rates of grade 3 or 4 adverse events (68%) and cardiac events (6%) were the same with and without trastuzumab.6
- In the GATSBY phase 2/3 trial, trastuzumab emtansine plus taxane was not superior to taxane alone in patients with previously treated, HER2-positive advanced gastric or GEJ cancer. Median OS was 7.9 months for the trastuzumab emtansine group and 8.6 months with taxane treatment (HR = 1.15; 95% CI, 0.87–1.51; one-sided P=0.86).7
- In a meta-analysis that incorporated data from the ToGA trial, the majority of studies (71%) found that HER2-positivity detected by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) was associated with poor survival.8
- A study evaluating the HER2 status of 48 patients with HER2-positive advanced gastric cancer who were treated with trastuzumab and chemotherapy found that 29.1% of tumors lost HER2 positivity on a post-progression biopsy. Data suggest that up to 35% of patients can have down-regulation of initially positive HER2 overexpression or amplification while receiving first-line trastuzumab.9
Pembrolizumab
A monoclonal antibody that blocks the binding of programmed (cell) death 1 (PD-1) on T cells to its ligand PD-L1, thereby reactivating an immune system response against tumor cells10,11
- In the open-label, phase 2 KEYNOTE-059 trial, pembrolizumab monotherapy was assessed in 259 patients with advanced gastric or GEJ cancer who had previously received at least two lines of treatment. The ORR was 11.6%, with complete response in 2.3% of the patients and a median duration of response of 8.4 months. Durable responses were observed in patients with PD-L1–positive and PD-L1–negative tumors. Pembrolizumab had a manageable safety profile.10
- The phase 3 KEYNOTE-061 trial recently failed to demonstrate a significant improvement in OS or PFS with pembrolizumab versus paclitaxel in patients with advanced gastric or GEJ cancer who progressed on first-line platinum and fluoropyrimidine chemotherapy. Median OS was 9.1 months with pembrolizumab and 8.3 months with paclitaxel (HR = 0.82; 95% CI, 0.66–1.03; one-sided P=0.0421). Median PFS was 1.5 months with pembrolizumab and 4.1 months with paclitaxel (HR = 1.27; 95% CI, 1.03–1.57). Analyses in protocol-specified subgroups showed a significant improvement in OS with pembrolizumab among patients with GEJ cancers as the primary tumor location. Pembrolizumab had a better safety profile than paclitaxel.11
References
- NCCN Guidelines. Gastric Cancer. Version 2.2019. National Comprehensive Cancer Network. org/professionals/physician_gls/pdf/gastric.pdf
- Barzi A, Hess LM, Zhu Y, et al. Real-world outcomes and patient (pt) characteristics for the second-line (2L) treatment of gastric, esophageal, or gastroesophageal junction (GEJ) adenocarcinoma (EGAC). J Clin Oncol. 2018;36(suppl): abstract e16011.
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235.
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39.
- Fuchs CS, Shitara K, Di Bartolomeo M, et al. RAINFALL: a randomized, double-blind, placebo-controlled phase III study of cisplatin (Cis) plus capecitabine (Cape) or 5FU with or without ramucirumab (RAM) as first-line therapy in patients with metastatic gastric or gastroesophageal junction (G-GEJ) adenocarcinoma. J Clin Oncol.2018;36(suppl 4): abstract 5.
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial. Lancet. 2010;376:687-697.
- Thuss-Patience PC, Shah MA, Ohtsu A, et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017;18:640-653.
- Jørgensen J, Hersom M. HER2 as a prognostic marker in gastric cancer—a systematic analysis of data from the literature. J Cancer.2012;3:137-144.
- Seo S, Ryu MH, Park YS, et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer. 2019;22:527-535.
- Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer. JAMA Oncol.2018;4:e180013.
