Hepatocellular Carcinoma – Treatments
Guidelines for Treatment of HCC
Given the hypervascular nature of hepatocellular carcinoma (HCC), arterially directed treatment is both intuitively appealing and clinically effective. Locoregional therapies used in the intermediate stage of HCC include transarterial embolization (TAE), transarterial chemoembolization (TACE), and transarterial radioembolization (TARE). In unresectable HCC, TACE compared with best supportive care (BSC) was found to result in significantly better survival (1 year = 57%, 2 years = 31%, and 3 years = 26%) vs BSC (1 year = 32%, 2 years = 11%, and 3 years = 3%).1 The challenge for the clinician is knowing when to transition from a locoregional approach to a systemic strategy, given the extent of disease.
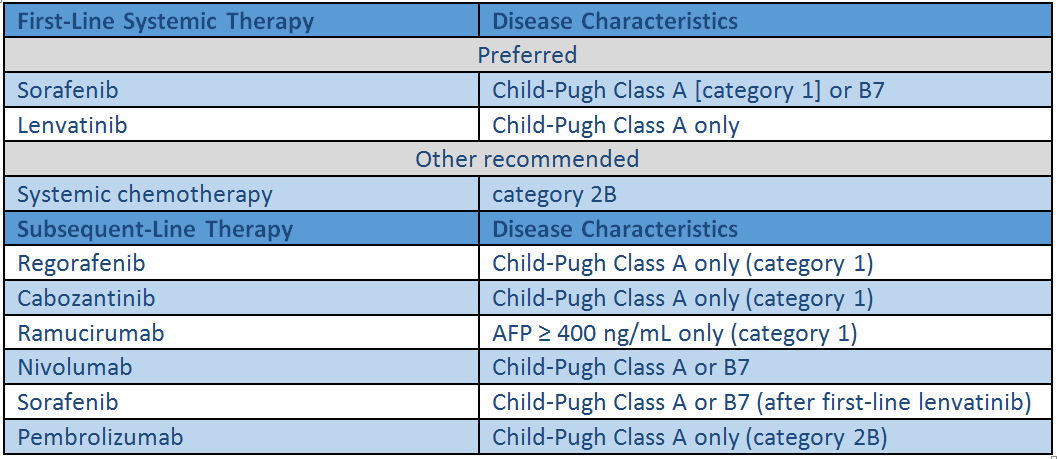
NCCN Recommended Systemic Therapies for Hepatocellular Carcinoma2
Clinical trial data
Sorafenib
An oral multi-kinase inhibitor that blocks the activity of several kinases involved in tumor-cell proliferation, angiogenesis, and apoptosis, including the serine-threonine kinases Raf-1 and B-Raf and the tyrosine kinase activity of vascular endothelial growth factor receptor (VEGFR) 1–3 and platelet-derived growth-factor receptor β (PDGFR-β)3
- In the phase 3 SHARP trial, 602 patients with advanced HCC who had not received previous systemic treatment had a median overall survival (OS) of 10.7 months in the sorafenib group and 7.9 months in the placebo group (hazard ratio [HR] = 0.69; 95% confidence interval [CI], 0.55–0.87; P<0.001). There was no significant difference between the two groups in the median time to symptomatic progression (4.1 months vs 4.9 months, respectively, P=0.77). The median time to radiologic progression was 5.5 months in the sorafenib group and 2.8 months in the placebo group (P<0.001). Seven patients (2%) in the sorafenib group and two patients (1%) in the placebo group had a partial response, while none of the patients had a complete response. Diarrhea, weight loss, hand-foot skin reactions, and hypophosphatemia were more common in the sorafenib group and were mainly mild to moderate in severity with similar discontinuation rates in the two study groups (38% vs 37%).4
- A subsequent analysis indicated that sorafenib consistently improved median OS and disease control rate (DCR) compared with placebo in patients with advanced HCC, irrespective of disease etiology, baseline tumor burden, performance status, tumor stage, and prior therapy.5
Lenvatinib
An oral tyrosine-kinase inhibitor (TKI) with multiple targets, including VEGFR 1–3, fibroblast growth factor receptor (FGFR 1–4), PDGFRα, and RET6
- The phase 3 REFLECT trial demonstrated non-inferiority of lenvatinib to sorafenib in previously untreated unresectable HCC (median OS = 13.6 vs 12.3 months; HR = 0.92). Treatment with lenvatinib resulted in significant improvements over sorafenib in progression-free survival (PFS), objective response rate (ORR), and time to progression. The most common adverse events were hypertension, proteinuria, dysphonia, and hypothyroidism with lenvatinib and palmar-plantar erythrodysaesthesia and diarrhea with sorafenib.6Based on these data, lenvatinib was approved by the FDA for first-line treatment of patients with unresectable HCC.
Ramucirumab
A monoclonal antibody that binds to the VEGFR-2 receptor and blocks binding of the ligands VEGF-A, VEGF-C, and VEGF-D, thereby inhibiting tumor angiogenesis7
- In the phase 3 REACH trial of 565 patients with advanced HCC following first-line treatment with sorafenib, ramucirumab failed to show a significant survival advantage compared with placebo (median OS = 9.2 vs 7.6 months, HR = 0.87; 95% CI, 0.72–1.05; P=0.14). A prespecified subset analysis suggested a survival benefit in patients with elevated AFP at baseline. A post-hoc subgroup analysis of the REACH trial revealed improved survival in patients with a baseline AFP serum level above 400 ng/mL compared with less than 400 ng/mL (median OS = 7.8 months vs 4.2 months; HR = 0.67; 95% CI, 0.51–0.9; P=0.006).7
- The phase 3 REACH-2 trial was designed to confirm the survival benefit of ramucirumab treatment in patients with baseline AFP ≥400 ng/mL observed in the REACH trial. In this trial, 292 patients with AFP ≥400 ng/mL were randomized 2:1 to intravenous (IV) ramucirumab 8 mg/kg every 2 weeks or IV placebo plus BSC. The median OS was 8.5 months with ramucirumab versus 7.3 months with placebo (HR = 0.710, P<0.0199). Median PFS was also significantly improved with ramucirumab compared with placebo (2.8 months vs 1.6 months; HR = 0.452; P<0.0001). Ramucirumab slightly increased the ORR (5% vs 1% for placebo; P=0.1697) and significantly increased the DCR (59.9% vs 38.9% for placebo; P=0.0006). Although ramucirumab was associated with more treatment-related adverse events, it was generally well tolerated.8
Regorafenib
An oral multikinase inhibitor9 that blocks the activity of RET, VEGFR1-3, PDGFR, BRAF, KIT and RAF-1
- In the phase 3 RESORCE trial of 573 adults with HCC who progressed on sorafenib and had Child-Pugh class A liver function, regorafenib improved OS with a HR of 0.63 compared with placebo (95% CI, 0.50–0.79; one-sided P<0.0001). Median survival was 10.6 months (95% CI, 9.1–12.1) for regorafenib versus 7.8 months (95% CI, 6.3–8.8) for placebo. The most common clinically relevant grade 3 or 4 treatment-emergent events were hypertension, hand-foot skin reaction, fatigue, and diarrhea.9
Cabozantinib
A multi-kinase inhibitor of MET, VEGFR1-3, RET, KIT, and AXL10
- The phase 3 CELESTIAL trial compared cabozantinib with placebo in 707 previously treated patients with advanced HCC. Median OS was 10.2 months for cabozantinib vs 8.0 months for placebo (HR for death = 0.76, 95% CI, 0.63–0.92; P=0.005). Median PFS was also statistically improved, from 5.2 months with cabozantinib vs 1.9 months with placebo (HR = 0.44, 95% CI, 0.36–0.52; P<0.0001). Adverse events observed in the cabozantinib versus placebo groups included hand-foot skin reaction (17% vs 0%), hypertension (16% vs 2%), increased aspartate aminotransferase (12% vs 7%), fatigue (10% vs 4%), and diarrhea (10% vs 2%), respectively.10
Nivolumab
A monoclonal antibody that blocks the binding of programmed (cell) death 1 (PD-1) on T cells to its ligand PD-L1, thereby reactivating an immune system response against tumor cells11
- The phase 1/2 CheckMate-040 trial was an open-label, non-comparative, dose escalation and expansion trial in 262 patients with advanced HCC and Child–Pugh A cirrhosis, who were either sorafenib naïve or had progression on sorafenib. In this trial, nivolumab had a manageable safety profile, an ORR of 20% in patients on 3 mg/kg (in the dose expansion phase), and an ORR of 15% (in the dose escalation phase). Baseline tumor cell PD-L1 expression and HCC etiology did not have an apparent effect on response rates.11
- In the phase III CheckMate-459 trial, nivolumab was compared with sorafenib as a first-line treatment in patients with unresectable HCC. The trial did not achieve statistical significance for its primary endpoint of OS (HR = 0.85; 95% CI, 0.72–1.02; P=0.0752).12
Pembrolizumab
An anti-PD-1 monoclonal antibody12 that blocks the binding of PD-1 on T cells to its ligand PD-L1, thereby reactivating an immune system response against tumor cells
- In the open-label, non-randomized, phase 2 KEYNOTE-224 trial, 104 patients with advanced HCC who had progressed on sorafenib were treated with pembrolizumab. ORRs were 17%, with 1 complete response and 17 (16%) partial responses. Median PFS was 4.9 months, and median OS was 12.9 months. The 12-month PFS and OS rates were 28% and 54%, respectively. Treatment-related adverse events occurred in 73% of the 104 patients.13
- The phase 3 KEYNOTE-240 trial investigated pembrolizumab plus BSC care for the treatment of patients with advanced HCC who were previously treated with systemic therapy. While there was an improvement in OS with pembrolizumab, it was not statistically significant compared with placebo plus BSC per the prespecified statistical plan (HR = 0.78; 95% CI, 0.611–0.998; P=0.0238). Results for PFS also favored pembrolizumab compared with placebo but did not reach statistical significance (HR = 0.78; 95%, 0.61–0.99; P=0.0209).14
References
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171.
- National Comprehensive Cancer Network (NCCN). Hepatobiliary cancers. Version 4.2019. December 20, 2019. www.nccn.org/professionals/physician_gls/PDF/hepatobiliary.pdf. Accessed December 20, 2019.
- Chang YS, Adnane J, Trail PA, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59:561-574.
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390.
- Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol.2012;57:821-829.
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173.
- Zhu AX, Park JO, Ryoo, BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol.2015;16:859-870.
- Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.2019;20:282-296.
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54-63.
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. 2017;389:2492-2502.
- Bristol-Myers Squibb. Bristol-Myers Squibb announces results from CheckMate-459 study evaluating Opdivo (nivolumab) as a first-line treatment for patients with unresectable hepatocellular carcinoma. June 24, 2019. https://news.bms.com/press-release/bmy/bristol-myers-squibb-announces-results-checkmate-459-study-evaluating-opdivo-nivol. Accessed December 20, 2019.
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952.
- Merck. Merck provides update on KEYNOTE-240, a phase 3 study of KEYTRUDA® (pembrolizumab) in previously treated patients with advanced hepatocellular carcinoma. February 19, 2019. www.mrknewsroom.com/news-release/oncology/merck-provides-update-keynote-240-phase-3-study-keytruda-pembrolizumab-previou. Accessed December 20, 2019.
